In numerous companies - especially in regulated industries such as medtech, food, pharmaceuticals, laboratories and automotive - a wide variety of evidence is required due to regulatory requirements. From simple reading, inspection or test certificates to training certificates and complex hygiene and safety checks - the requirements are varied and demanding. It is important to maintain an overview of open and completed certificates at all times in order to be able to efficiently and reliably provide evidence of implementation to authorities, control bodies and regulators at any time.
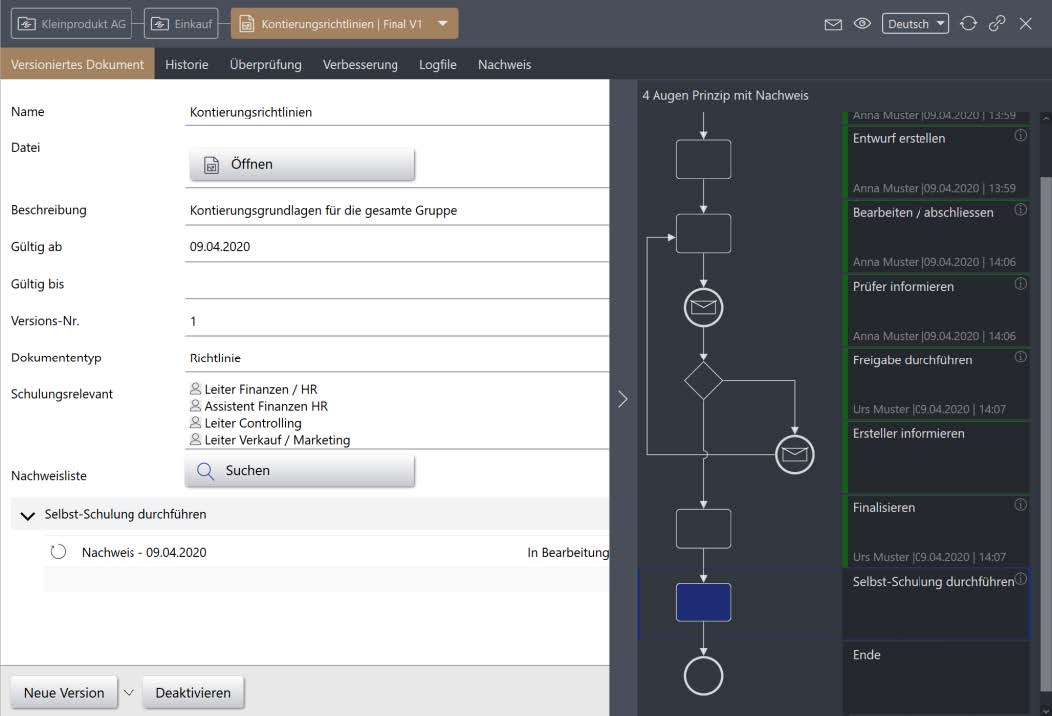
The IMS solution “Verification provision” enables the systematic recording and documentation of any verification variants throughout the company. Thanks to the end-to-end digital processes, clarity is created as to who or what was trained, checked or tested in the company at what time and on what topic. The verification function is linked to an electronic workflow. The workflow steps can be configured as required and adapted to specific needs.
Core functions of the “Process execution verification” solution
- Complete traceability (who planned the verification / training)
- Person to be trained is selected via job, group or person
- Information about the open task is sent by e-mail and/or dashboard
- Up-to-date overview of open training courses at all times
- Proof is provided per document / process version (with time stamp per employee)
- At the touch of a button: personal overview of open training courses / completed certificates
- Simple recording and planning of follow-up training and inspections
- Set a deadline: Set a date by which the training must be completed
- Reminder function: if a certificate is not completed on time, the system sends a reminder. Further escalation level: inform responsible person / supervisor
- Can be combined with freely definable process execution and with 4- or 6-eye workflow for document / process control